Australian-led research is developing an easy repair rubber and catalyst combo.
Self-Repairing Rubber Comes a Step Closer in
Scientists have long been investigating the prospects of repairing rubber, and now an Australian project believes that it may have a solution that will help rubber products last longer and thus, reduce recycling levels.
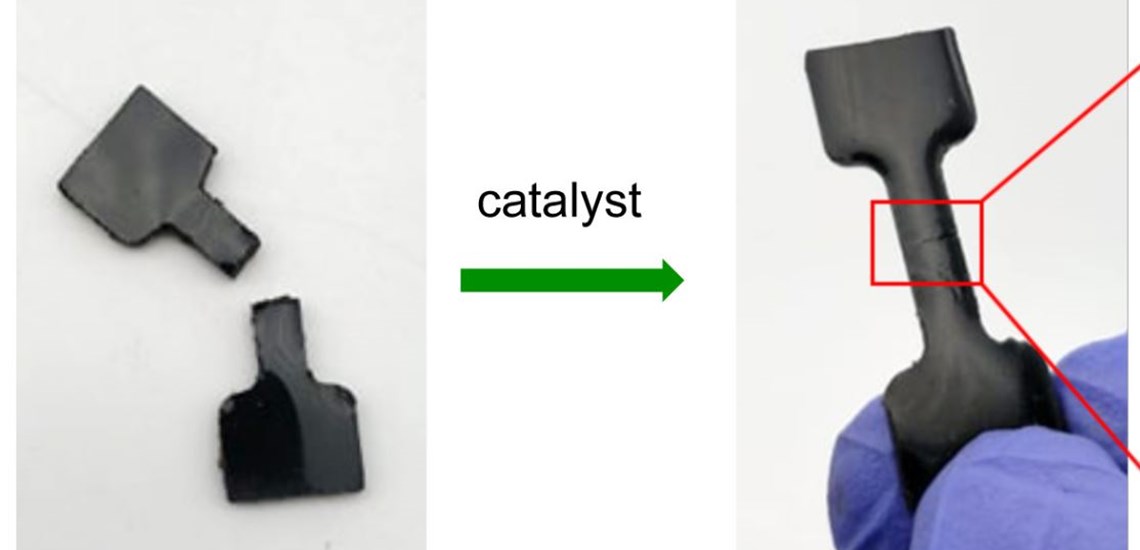
Researchers have developed a new kind of rubber and catalyst that together can be used with low energy consumption to make flexible, repairable, sustainable objects – including car tyres.
The new rubber material, made from cheap and plentiful industrial waste products sulphur, canola cooking oil and dicyclopentadiene (DCPD) from petroleum refining, can be completely repaired and returned to its original strength in minutes – even at room temperature – with an amine catalyst.
The new type of rubber can be seamlessly repaired if damaged, and can also be recycled, says research leader Flinders University Associate Professor Justin Chalker, whose team’s breakthrough findings are described in leading international journal Chemical Science.
The amine catalyst used to trigger the reaction that causes the rubber to self-repair occurs within minutes in some cases, and it is all done at room temperature, scientists say.
“This study reveals a new concept in the repair, adhesion and recycling of sustainable rubber,” says Associate Professor Chalker, adding too many plastics, rubbers and ceramics are not recyclable.
“It is exciting to see how the underlying chemistry of these materials has such wide potential in recycling, next-generation adhesives, and additive manufacturing,” Associate Professor Chalker says.
Researchers from the Chalker Lab at the Flinders University Institute for Nanoscale Science and Technology, with the University of Liverpool and University of Western Australia colleagues, say the new rubber can be used as a “latent adhesive”.
“The rubber bonds to itself when the amine catalyst is applied to the surface. The adhesion is stronger than many commercial glues,” says University of Liverpool researcher Dr Tom Hasell. “The rubber is not ‘sticky’ until the catalyst is applied.”
“The polymer is also resistant to water and corrosion.”